Clemson University
Community for the latest research in new membrane materials.
Past work has shown that regenerated cellulose (RC) membranes can be functionalized with polymer nanolayers via atom transfer radical polymerization (ATRP) to produce high capacity protein adsorbers. These functionalized membranes have shown competitive binding capacities at a drastically increased throughput compared to the costly industry standard resin chromatography media.
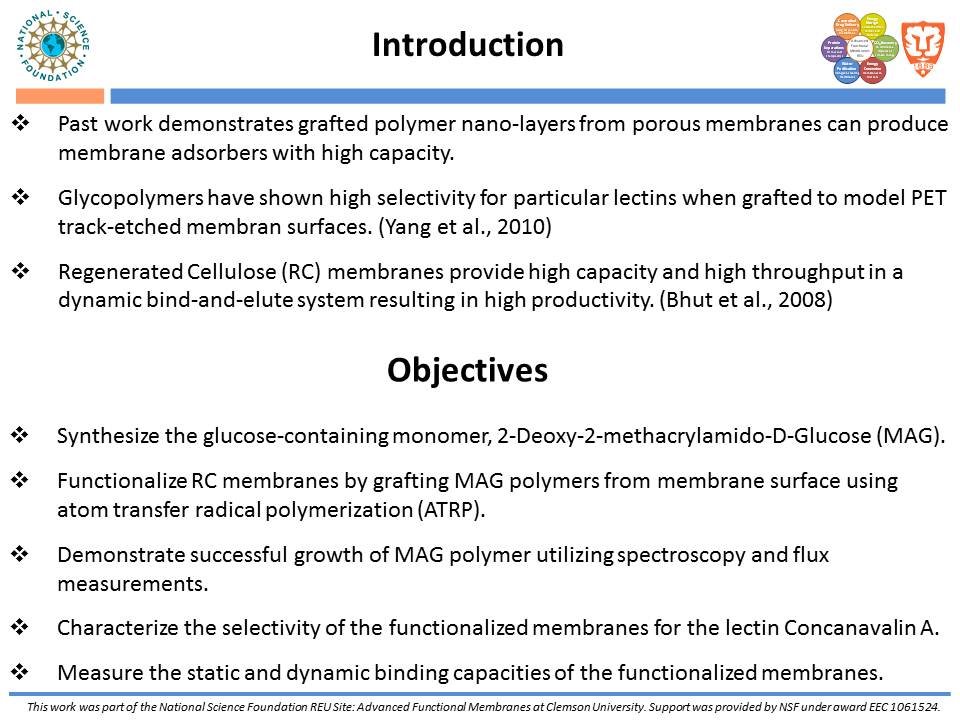
The affinity ligand project focuses on a novel method for the purification of lectins. From past work, glycopolymers have shown high selectivity for particular lectins when grafted to model PET track-etched membrane surfaces. Glycopolymer chains will be grafted from a regenerated cellulose substrate to produce a polymer nano-layer. The open pore structure of the cellulose membranes provides both high capacity and high throughput, resulting in increased productivity.
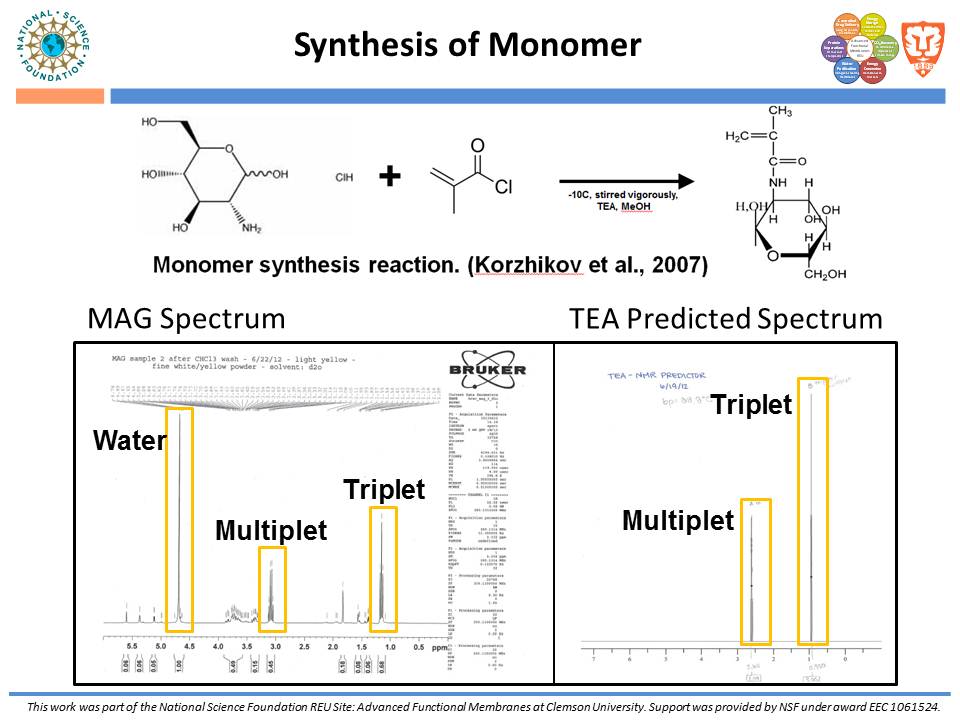
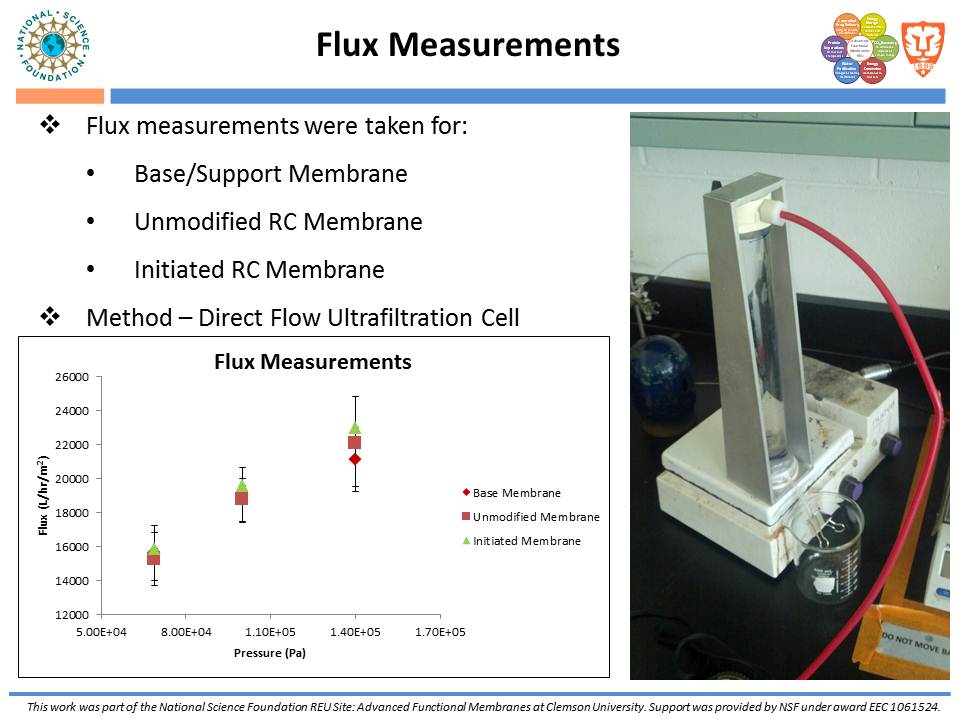
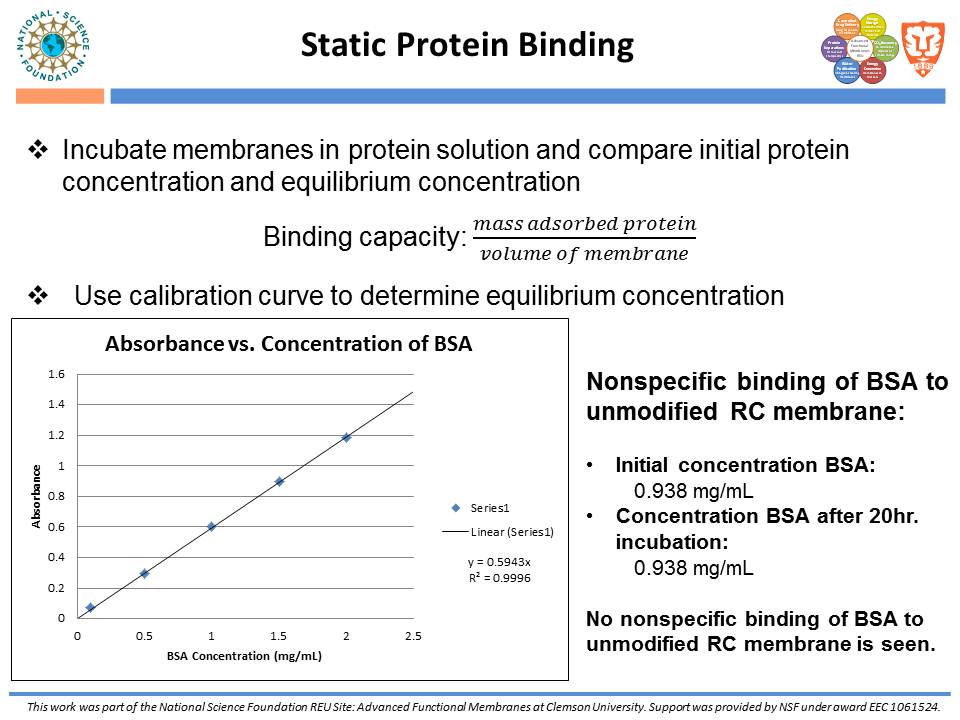
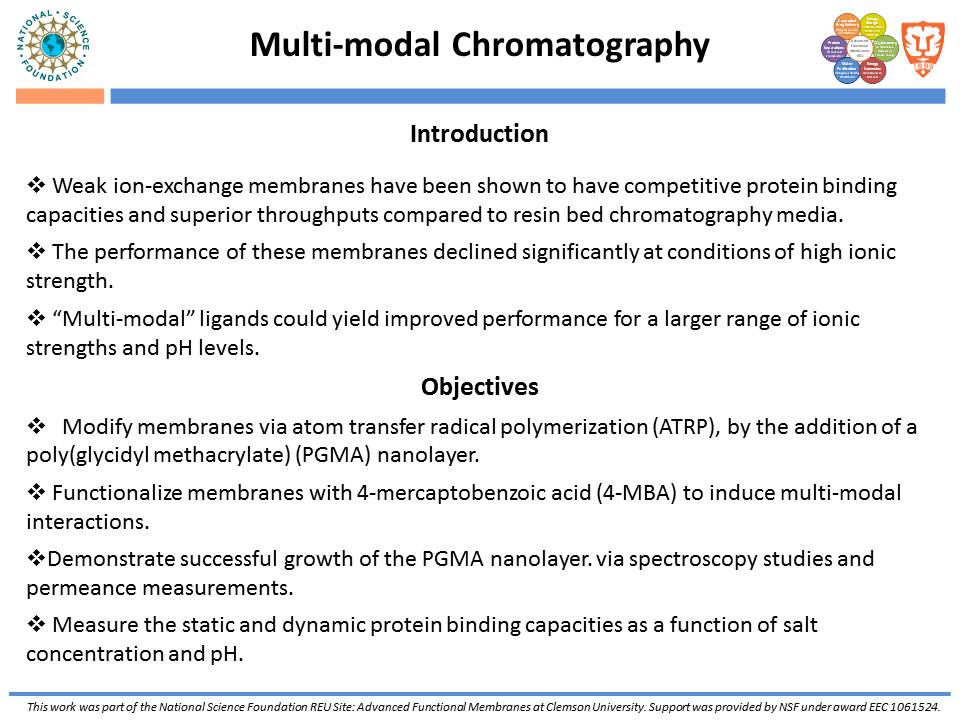
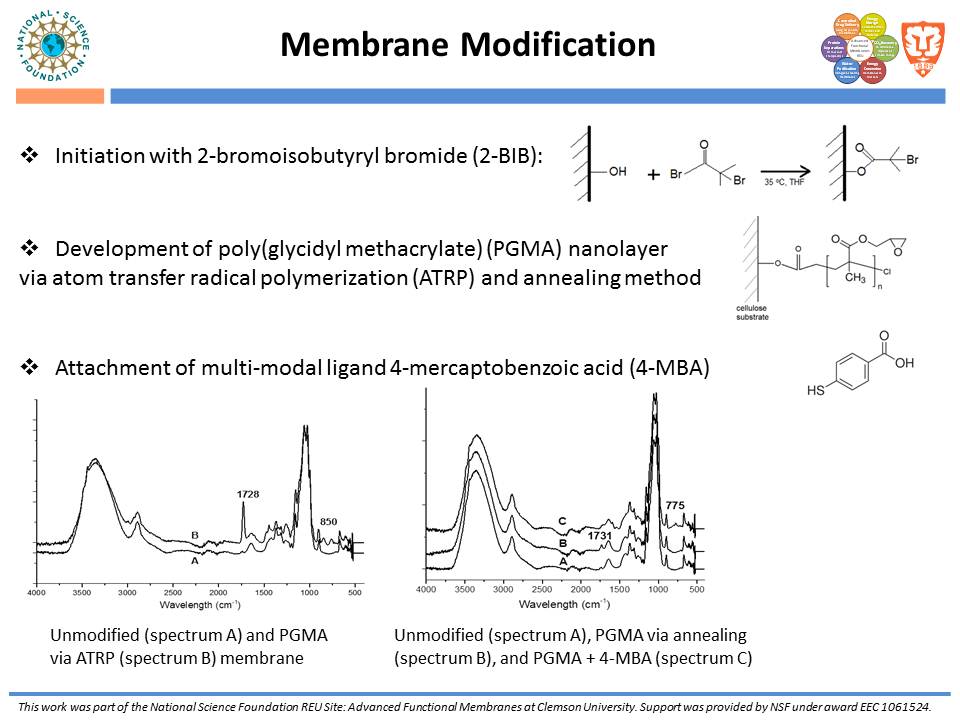
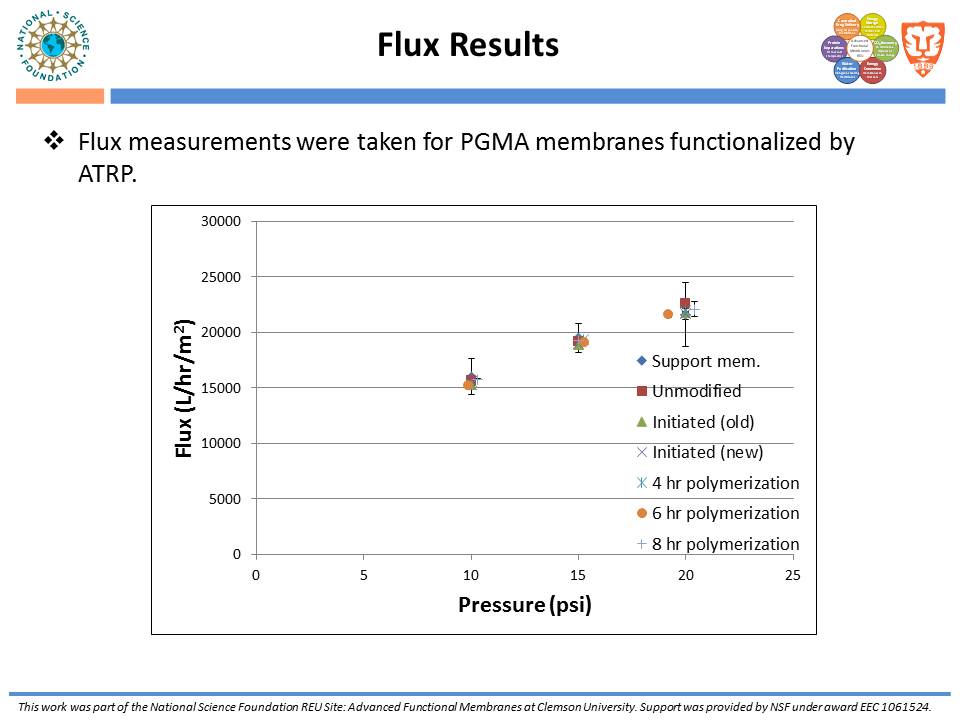
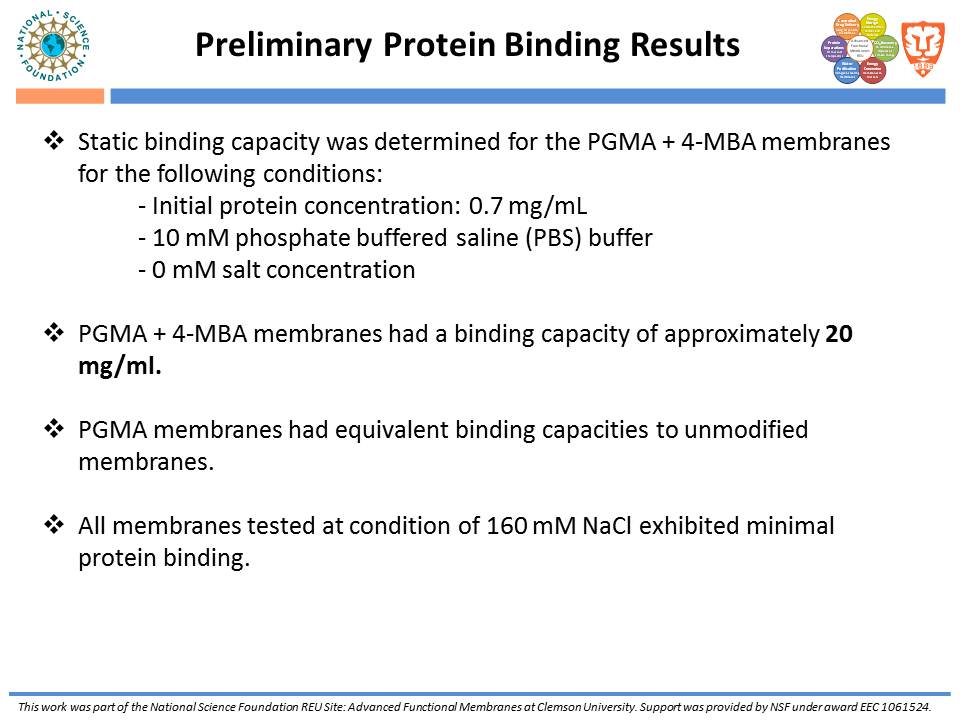
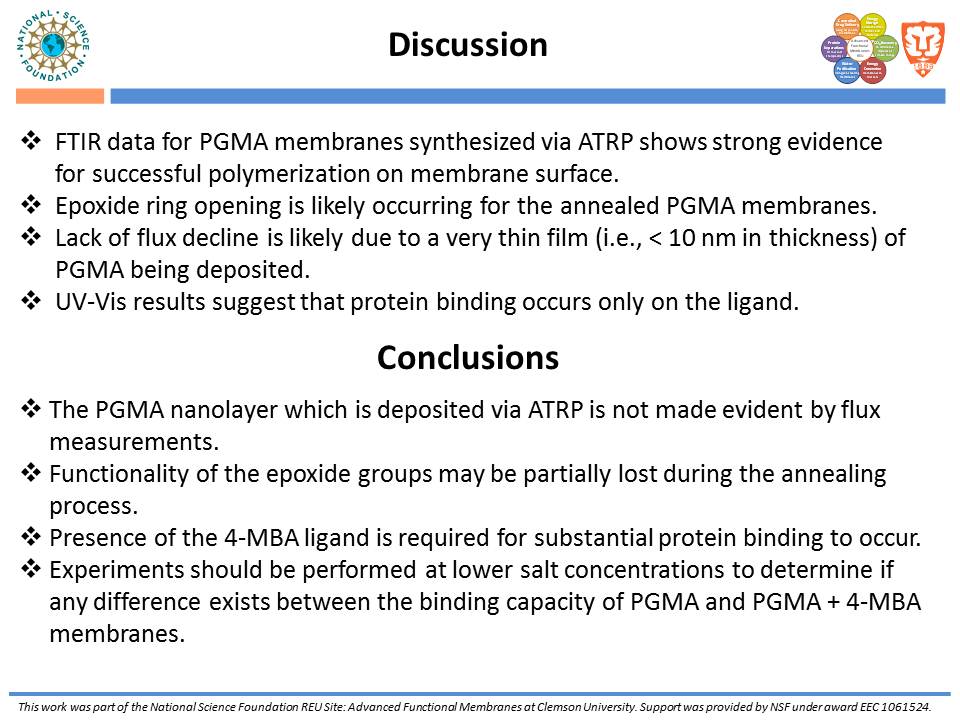
While traditional ion-exchange membrane adsorbers, modified by ATRP, have been successful at weak ionic strengths, their performance declines significantly at higher ionic strengths. Membranes functionalized with “multi-modal” ligands (i.e., ligands capable of multiple types of protein-ligand interactions) could exhibit a larger operating range than traditional ion-exchange membranes. Membranes were prepared by using the monomer, glycidyl methacrylate (GMA), and grafting the polymer from the regenerated cellulose substrate via ATRP. The resulting nanolayer was further functionalized by adding 4-mercaptobenzoic acid (4-MBA), capable of multi-modal interaction, to the epoxide portions of the poly-glycidyl methacrylate (PGMA) polymer. After each functionalization, attenuated total reflectance Fourier transform infrared (ATR-FTIR) analyses were performed. These IR spectra demonstrated successful polymer growth and addition of the multi-modal ligand. Direct-flow water flux and static protein binding capacity were measured.
Information
- Affinity and Multimodal Membranes
- Poster Sessions1
- Images10
- Last UpdatedJuly 30, 2012 2:58pm EDT
Contacts
-
Phil Langford

REU Program 2012
Texas A&M
College Station, Texas