Clemson University
Community for the latest research in new membrane materials.
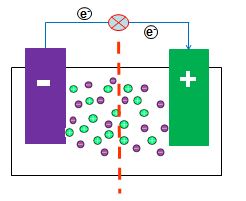
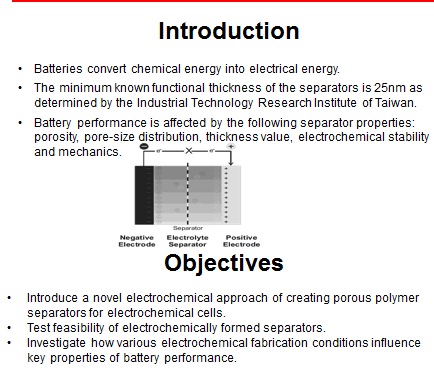
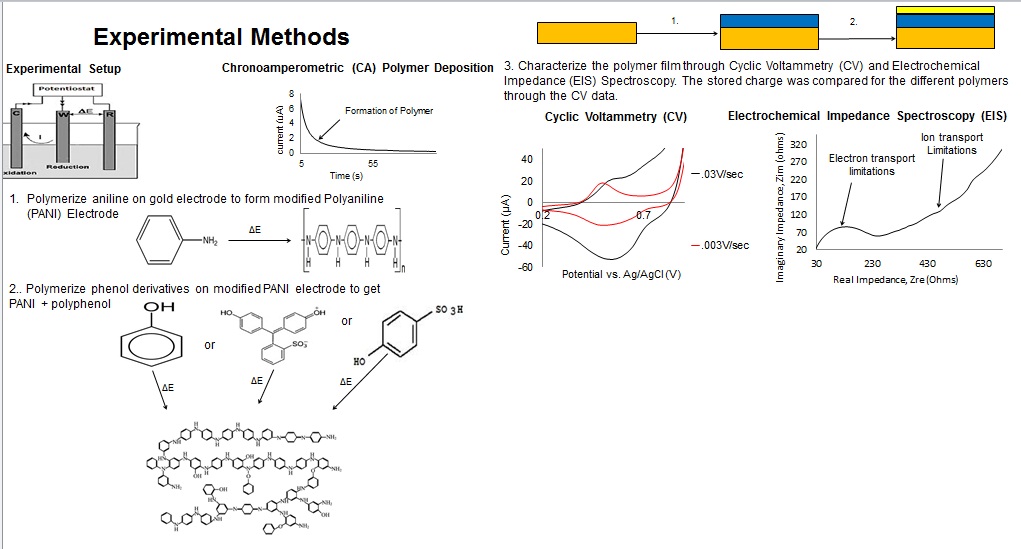
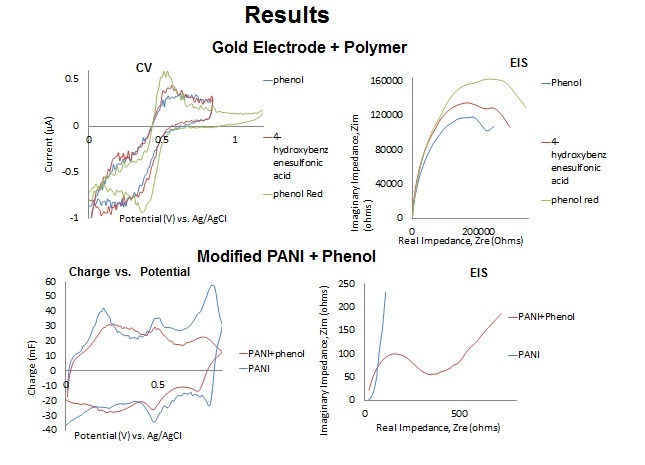
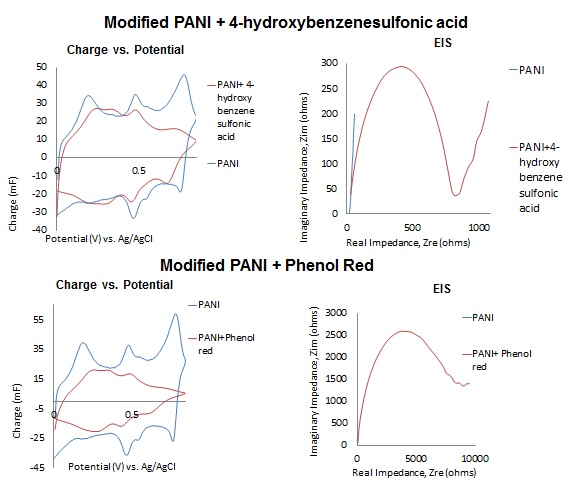
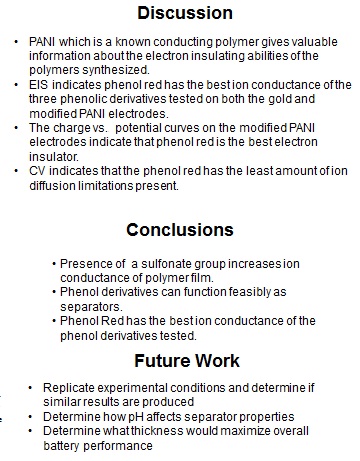
Battery separators are membranes that prevent the direct contact of the positive and negative terminals of a battery while allowing ion conductance and preventing electron flow. Battery performance is affected by the following separator properties: porosity, pore-size distribution, thickness value, electrochemical stability and mechanics. In this project, polymer ion-exchange membranes (IEMs) were synthesized in order to determine the feasibility of using electrochemically synthesized film separator layers in batteries. Electrochemical polymerization on Polyaniline modified gold electrodes was utilized in order to synthesize functional IEMs of varying thickness and functionality using a solution of electrolytes and phenolic derivatives. The polymer films were then characterized using redox active compounds to determine the functional feasibility of the separator layers.
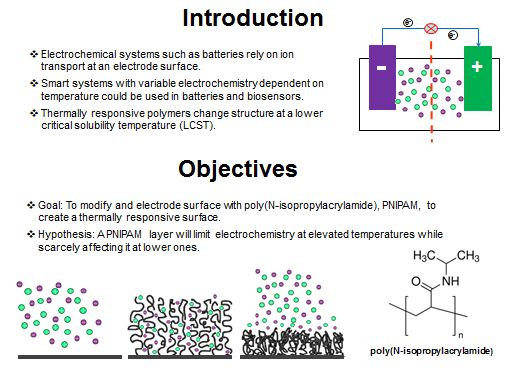
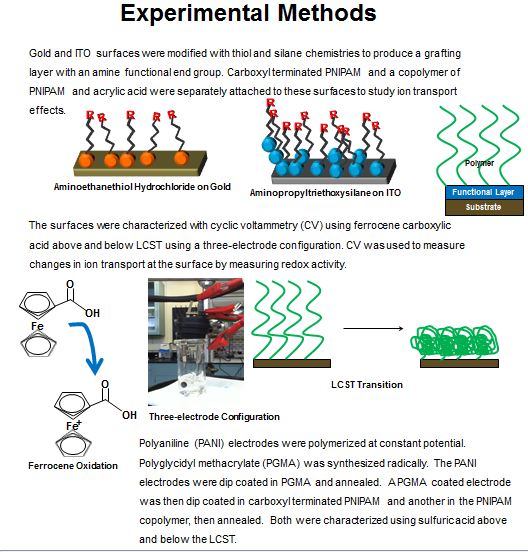
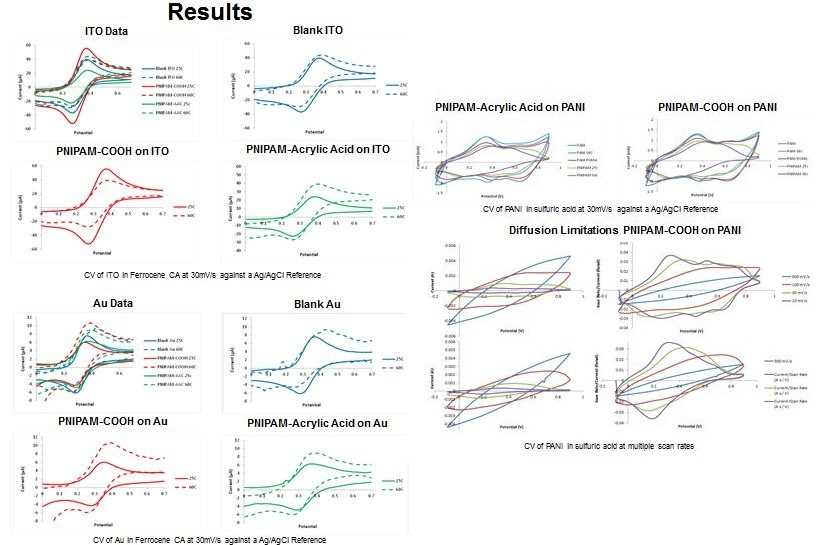
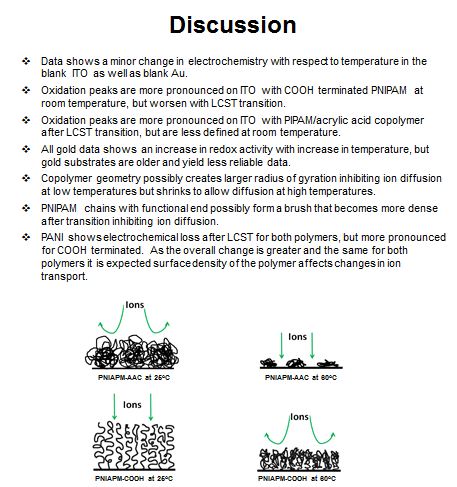
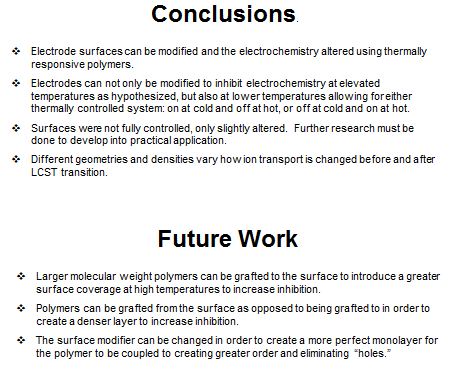
This project tests the modification of electrode surfaces with the thermally responsive polymer, poly(N-isopropylacrylamide), to selectively impede the ion transport to and from the electrode surface. Gold, indium tin oxide, and polyaniline electrodes were characterized with cyclic voltammetry at low and high temperatures to measure the redox activity.
Information
- Battery Separator Layers
- Poster Sessions1
- Images10
- Documents2
- Last UpdatedFebruary 19, 2025 10:01pm EST
Contacts
-
Mathew Boyer

REU Program 2012
Clemson University
-
Amit Jha

REU Program 2012
Clemson University